| Login | ||

Healthcare Training Institute - Quality Education since 1979
CE for Psychologist, Social Worker, Counselor, & MFT!!

Section 6
Root Cause Analysis
Question 6
| Test
| Table of Contents
According to the World Health Organization definition, pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse reactions or any other drug-related problem [1].
In 1999, the US Institute of Medicine’s report ‘To err is human’ showed that there were more than one million preventable adverse drug reactions each year in the USA, of which 44 000–98 000 were fatal and 7000 were due to medication errors [3]. Although pharmacovigilance had always been concerned with minimizing the risks of adverse drug reactions and medication errors, in March 2007 the Erice Manifesto formulated a new vision, in which patient safety constitutes one of the main challenges to pharmacovigilance [4].
Root Cause Analysis
Root cause analysis is a systematic investigation technique that looks beyond the affected individual and seeks to understand the underlying causes and environmental context in which an incident related to a medication error occurred. It is usually applied to serious adverse events or critical incidents, which are also known as sentinel events.
A sentinel event is an unexpected occurrence that involves death or serious physical or psychological injury, or a risk thereof [9, 10]. The phrase ‘or a risk thereof’ includes any process variation a recurrence of which would carry a significant chance of a serious adverse outcome. Such events are called sentinel events because they signal the need for immediate investigation and response, as the term ‘signal’ implies in pharmacovigilance [11].
There are several methods of conducting root cause analysis, such as the Canadian root cause analysis framework, the Ishikawa or Fishbone Diagram [12] and the Guidelines for Root Cause Analysis of the Massachusetts Medical Society [13], but they all have the same goals and the same concepts.
The Moroccan Pharmacovigilance Centre has taken action to prevent medication errors after root cause analysis, using the Massachusetts Medical Society’s method in 30 cases of local reactions to intravenous flucloxacillin, with tissue necrosis leading to amputation in two cases. This method has four steps: describing the event, identifying the proximate cause(s) that led to the effect(s), identifying the contributing factors (or latent errors) that led to the proximate cause(s), and creating an action plan.
Root cause analysis identified the proximate cause and the contributory factors: failure to follow the recommendations of the Summary of Product Characteristics (SPC) and the absence of water for intravenous injection in the drug box [14].
Prevention
The duties of pharmacovigilance centres in preventing adverse drug reactions and medication errors include alerting healthcare professionals to the importance of reporting such errors, making them aware of the factors that cause them, encouraging them to develop a safety culture that leads to enhanced awareness, and stressing the need for commitment among healthcare professionals in preventing medication errors and improving patient care.
In some countries the two functions of pharmacovigilance and drug regulation reside in the same organization (for example, the Medicines and Healthcare products Regulatory Agency in the UK). Elsewhere, collaboration between pharmacovigilance centres and regulatory agencies is important. For example, after receiving, detecting, and analyzing notifications of suspected adverse drug reactions, the Moroccan Pharmacovigilance Centre submits these results to the Moroccan Drug Regulatory Directorate [18], which can submit the findings and proposed solutions to the national commission of pharmacovigilance, which submits the outcome to the Minister of Health for a final decision.
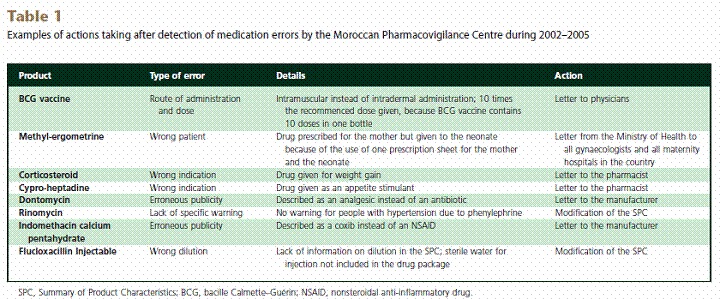
Conclusion
Pharmacovigilance centres can contribute to the detection and prevention of medication errors. Collaboration between poison control centres and pharmacovigilance centres needs to be strengthened, in order to improve the quality of data collected, enhancing patient safety, and bridges need to be built linking pharmacovigilance centres, poison control centres, and organizations dedicated to patient safety, in order to avoid duplication of workload.
--Bencheikh, R., & Benabdallah, G. (2009). Medication errors: pharmacovigilance centres in detection and prevention. British Journal Of Clinical Pharmacology, 67(6), 687-690. doi:10.1111/j.1365-2125.2009.03426.x
Personal
Reflection Exercise #6
The preceding section contained information
about root cause analysis and sentinel events. Write one case study example
regarding how you might use the content of this section in your practice.
Reviewed 2023
Update
Pharmacovigilance: reporting requirements throughout a product's lifecycle
Lucas, S., Ailani, J., Smith, T. R., Abdrabboh, A., Xue, F., & Navetta, M. S. (2022). Pharmacovigilance: reporting requirements throughout a product's lifecycle. Therapeutic advances in drug safety, 13, 20420986221125006. https://doi.org/10.1177/20420986221125006
Peer-Reviewed Journal Article References:
Halfond, R. W., Wright, C. V., & Bufka, L. F. (2021). The role of harms and burdens in clinical practice guidelines: Lessons learned from the American Psychological Association's guideline development. Clinical Psychology: Science and Practice, 28(1), 19–28.
McKay, D., & Jensen-Doss, A. (2021). Harmful treatments in psychotherapy. Clinical Psychology: Science and Practice, 28(1), 2-4.
Teachman, B. A., White, B. A., & Lilienfeld, S. O. (2021). Identifying harmful therapies: Setting the research agenda. Clinical Psychology: Science and Practice, 28(1), 101–106.
QUESTION 6
What is a sentinel event? To select and enter your answer go to Test.
Test
Section 7
Table of Contents
Top
Excerpts from Bibliography referenced in this article
1. World Health Organization. The Importance of Pharmacovigilance, Safety Monitoring of Medicinal Products. Geneva:WHO, 2002.
3. Khon LT, Corrigan JM, Donaldson MS, eds. To Err is Human. Building a Safer Health System.Washington DC: National Academy Press, 1999.
4. Anonymous. The Erice Manifesto. For global reform of the safety of medicines in patient care. Drug Saf 2007; 30: 187–90.
9. Haas D. Sentinel events. In memory of Ben – a case study. Jt Comm Perspect 1997; 17: 12–5.
10. Anonymous. Sentinel events: approaches to error reduction and prevention. Jt Comm J Qual Improv 1998; 24: 175–86.
11. Hauben M, Aronson JK. Defining ‘signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf 2009; 32: 99–110.
12. Ishikawa K.What is Total Quality Control? The Japanese Way. Englewood Cliffs, NJ: Prentice Hall, 1985.
13. Massachusetts Medical Society. Patient safety: conducting a root cause analysis of adverse events. Available at
http://www.massmed.org/AM/Template.cfm?Section=Patient_Safety_Conducting_a_Root_Cause_Analysis_of_Adverse_Events (last accessed 28 February 2009).
14. Benkirane RR, R-Abouqal R, Haimeur CC, El Kettani SEC,
Azzouzi AA, Alaoui AAM, Thimou AA, Nejmi MM, Maazouzi WW,Madani NN, Edwards IR, Soulaymani RR. Incidence of adverse drug events and medication errors in Intensive Care Units: a prospective multicenter study. J Patient Saf 2009; 5: 16–22.
18. Royaume de Maroc.Ministère de la Santé. Bonnes pratique de pharmacovigilance. Centre Marocain de Pharmacovigilance, 28 Janvier 2004. Available at http://www.capm.ma/sources_site_capm/pv_site_capm/1_ BONNES%20PRATIQUES%20DE%20PHARMACOVIGILANCE_MAROC.pdf (last accessed 28 February 2009)

